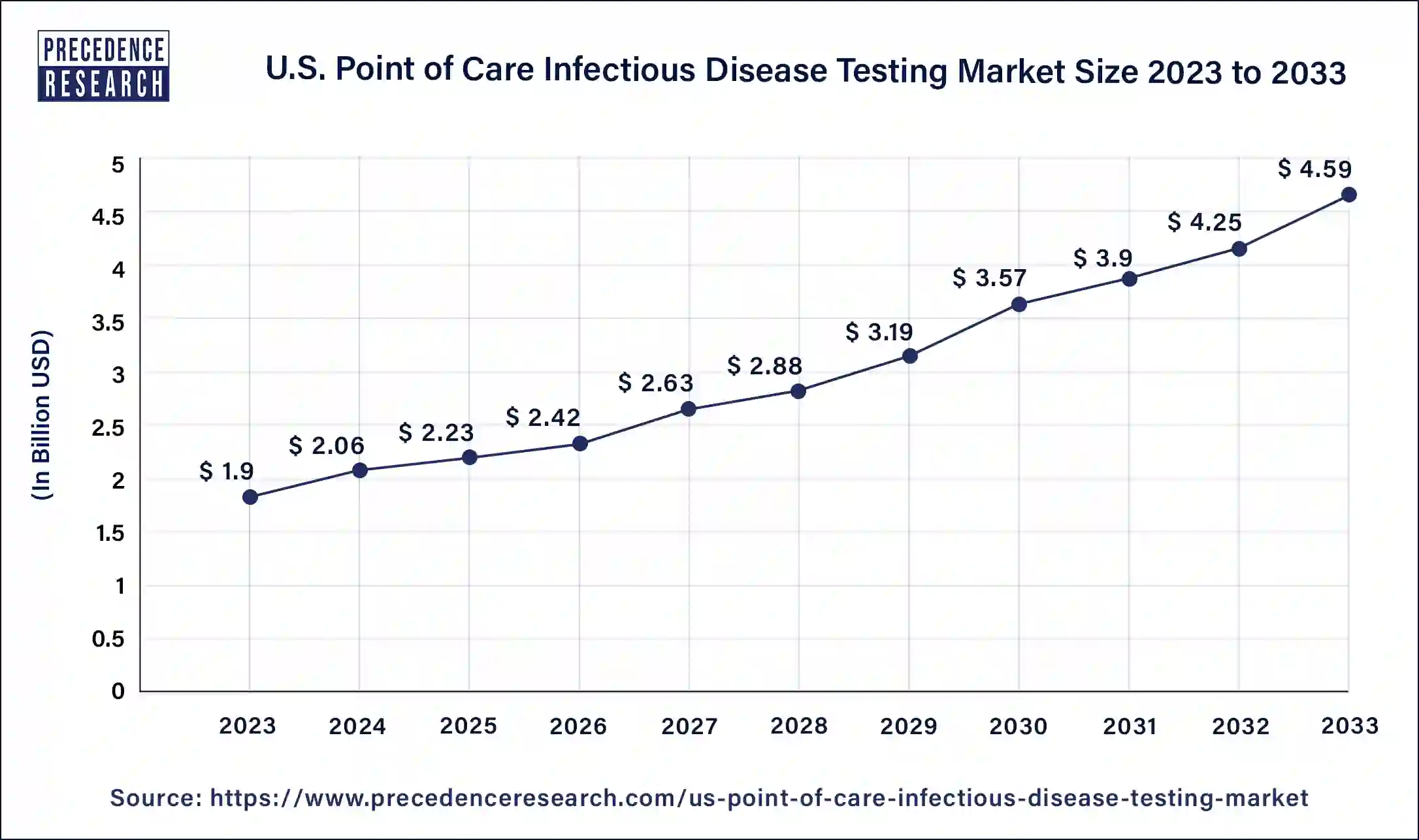
The U.S. point of care infectious disease testing market size was evaluated at USD 1.9 billion by 2023 and is projected to gain around USD 4.59 billion by 2033 with a CAGR of 9.30% between 2024 and 2033.
Key Points
- By disease, the influenza/flu segment has held a major revenue share of 24.42% in 2023.
- By disease, the respiratory syncytial virus (RSV) segment is the second largest in the market in 2023.
- By end-user, the hospitals segment dominated market with the largest revenue share of 38.56% in 2023.
Market Overview
The U.S. Point of Care Infectious Disease Testing Market refers to the market for diagnostic tests conducted at or near the site of patient care rather than in a centralized laboratory. These tests are crucial for the rapid detection and management of infectious diseases such as influenza, HIV, hepatitis, and others. The market encompasses a variety of testing methods including immunoassays, molecular diagnostics, and rapid antigen tests, which offer quick results and facilitate immediate clinical decisions.
Get the Sample Copy of This Report@ https://www.precedenceresearch.com/sample/4461
Growth Factors
Several factors drive the growth of the U.S. Point of Care Infectious Disease Testing Market. Firstly, the increasing prevalence of infectious diseases necessitates rapid and accurate diagnostic tools to facilitate prompt treatment and containment efforts. Additionally, advancements in technology have led to the development of more sensitive and specific point-of-care testing devices, enhancing their reliability and usability. Moreover, the growing demand for decentralized testing solutions that reduce turnaround times and improve patient outcomes is fueling market expansion.
U.S. Point of Care Infectious Disease Testing Market Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 1.9 Billion |
| Market Size in 2024 | USD 2.06 Billion |
| Market Size by 2033 | USD 4.59 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 9.30% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Disease and End-user |
Market Dynamics
Drivers
Key drivers of growth in this market include the rising adoption of point-of-care testing by healthcare providers to streamline workflow and enhance patient care efficiency. The shift towards personalized medicine and targeted therapies also drives demand for rapid diagnostic solutions that enable tailored treatment approaches. Furthermore, government initiatives promoting early diagnosis and management of infectious diseases contribute significantly to market growth by encouraging the adoption of point-of-care testing technologies.
Opportunities
The U.S. Point of Care Infectious Disease Testing Market presents numerous opportunities for market players. Expansion opportunities exist in developing innovative testing platforms that integrate multiple diagnostic functionalities into compact and user-friendly devices. Additionally, expanding market penetration in non-traditional settings such as pharmacies, urgent care clinics, and home healthcare settings offers promising avenues for growth. Furthermore, strategic partnerships and collaborations between healthcare providers and diagnostic companies can facilitate market expansion and product innovation.
Challenges
Despite its growth prospects, the market faces several challenges. Regulatory complexities associated with obtaining approvals for point-of-care testing devices can hinder market entry and product commercialization timelines. Quality control issues related to maintaining accuracy and reliability of tests across different settings pose significant challenges. Moreover, the need for substantial investments in research and development to continuously improve testing technologies and address emerging infectious diseases presents financial challenges to market players.
Region Insights
Geographically, the U.S. dominates the Point of Care Infectious Disease Testing Market owing to its advanced healthcare infrastructure, high healthcare expenditure, and favorable reimbursement policies. The presence of key market players and extensive research activities further contribute to market growth in this region. Additionally, the increasing focus on early disease detection and rapid diagnostic capabilities within the U.S. healthcare system enhances the adoption of point-of-care testing solutions across various healthcare settings.
Read Also: https://www.businesswebwire.com/credit-card-payments-market/
U.S. Point of Care Infectious Disease Testing Market Companies
- Beckton Dickinson
- Roche
- Biomerieux
- Siemens
- Bio-rad
- Chembio Diagnostic Systems
- Quidel Corporation
- Abbott
Recent Developments
- In March 2024, in spring 2024, the City University of New York (CUNY) Institute for Implementation Science in Population Health (ISPH) and the CUNY Graduate School of Public Health and Health Policy (CUNY SPH) will launch a critical two-year prospective epidemiologic study in partnership with Pfizer to monitor acute respiratory infections nationwide.
- In February 2022, The World Health Organization (WHO) approved Trinity Biotech plc’s new HIV screening tool, Trin Screen HIV.
Segments Covered in the Report
By Disease
- Pneumonia Or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV)
- TB and Drug Resistant TB POC
- Influenza/Flu POC
- HIV POC
- Others
By End-user
- Hospitals
- Clinics
- Home
- Assisted Living Healthcare Facilities
- Laboratories
- Others
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.dailystatsnews.com/